Mechanism of excited state deactivation of indan-1-ylidene and fluoren-9-ylidene malononitriles†
Abstract
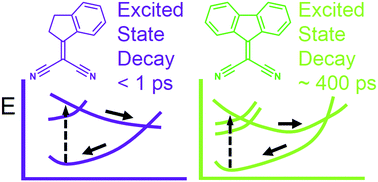
Herein, we report complementary computational and experimental evidence supporting the existence, for indan-1-ylidene malononitrile and fluoren-9-ylidene malononitrile, of a non-radiative decay channel involving double bond isomerisation motion. The results of UV-Vis transient absorption spectroscopy highlight that the decay takes place within hundreds of picoseconds. In order to understand the related molecular mechanism, photochemical reaction paths were computed by employing multiconfigurational quantum chemistry. The results indicate that the excited state deactivation occurs via concerted double bond twisting of the dicyanovinyl (DCV) unit coupled with a pyramidalisation of its substituted carbon. It is also shown that the observed differences in the excited state lifetimes when passing from indan-1-ylidene malononitrile to fluoren-9-ylidene are associated with the change in the topography of the conical intersection driving the decay from intermediate to sloped, respectively.


 Please wait while we load your content...
Please wait while we load your content...