Promoting oxygen vacancy formation and p-type conductivity in SrTiO3via alkali metal doping: a first principles study†
Abstract
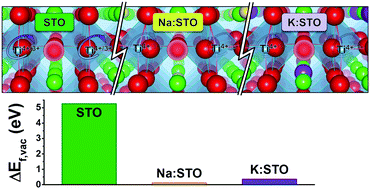
Strontium titanate (SrTiO3, STO) is a prototypical perovskite oxide, widely exploited in many technological applications, from catalysis to energy conversion devices. In the context of solid-oxide fuel cells, STO has been recently applied as an epitaxial substrate for nano-sized layers of mixed ion–electron conductive catalysts with enhanced electrochemical performances. To extend the applications of such heterogeneous nano-cathodes in real devices, also the STO support should be active for both electron transport and oxide diffusion. To this end, we explored using first-principles calculations the strategy of doping of STO at the Sr site with sodium and potassium. These two ions fit in the perovskite structure and induce holes in the STO valence band, so as to obtain the desired p-type electronic conduction. At the same time, the doping with alkali ions also promotes the formation of oxygen vacancies in STO, a prerequisite for effective oxide diffusion. Analysis of electron density rearrangements upon defect formation allows relating the favorable vacancy formation energies to an improved electronic delocalization over the oxide sub-lattice, as observed in closely related materials (e.g. Sr2Fe1.5Mo0.5O6). Overall, our results suggest the alkali-doped STO as a new potential substrate material in nanoscale heterogeneous electrodes for solid oxide electrochemical cells.


 Please wait while we load your content...
Please wait while we load your content...