Enhanced photoelectrochemical water splitting efficiency of hematite electrodes with aqueous metal ions as in situ homogenous surface passivation agents†
Abstract
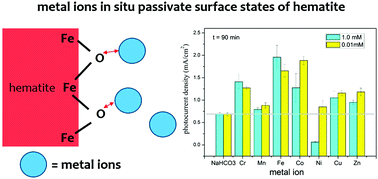
Passivation of surface states is known to reduce the onset photocurrent potential by removing the Fermi level pinning effect at the Helmholtz layer and enhance the photocurrent plateau by suppressing recombination loss in the space charge region. We report for the first time that metal ions can effectively passivate surface states in situ that improves the photoelectrochemical (PEC) performance of hematite electrodes. Among metal ions studied, Cr(III), Mn(II), Fe(II), Co(II), Cu(II) and Zn(II) were found to enhance the photocurrent by 30–300%; whereas photocurrent density significantly dropped by 90% in Ni(II) solution after 90 min of illumination. We further hypothesized that the surface states might be the high affinity adsorption sites on hematite surfaces. Once the surface states are occupied by metal ions, along with the Schottky barrier effect at the hematite/electrolyte interface formed by adsorbed metal ions, the PEC performance is enhanced. Our results also enable the design of a potential PEC based water treatment method to extract additional energy, for example, in the brines (containing concentrated metal ions and electrolyte) of membrane processed wastewater.


 Please wait while we load your content...
Please wait while we load your content...