An alternative explanation of the cononsolvency of poly(N-isopropylacrylamide) in water–methanol solutions
Abstract
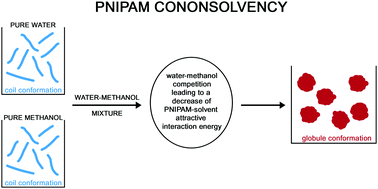
Cononsolvency refers to the experimental finding that poly(N-isopropylacrylamide), PNIPAM, has a coil conformation in both pure water and pure methanol, at 20 °C and 1 atm, but assumes a globule conformation in methanol–water solutions, over the 0.1 ≤ X(MeOH) ≤ 0.4 methanol molar fraction. This strange phenomenon has recently been rationalized by claiming that: (a) MeOH molecules are able to bind two distant monomers in the chain, driving collapse [Nat. Commun., 2014, 5, 4882]; (b) the preferential binding of MeOH stabilizes globule conformations due to a conformational entropy gain of the chain [J. Phys. Chem. B, 2015, 119, 15780]. In the present work a self-consistent application of the approach already used to rationalize the effect of sodium salts, urea and tetramethylurea on PNIPAM collapse [Phys. Chem. Chem. Phys., 2015, 17, 27750; 2016, 18, 14426] leads to a different explanation. The emerging scenario is that cononsolvency is caused by the fact that, on adding methanol, the competition between water and methanol molecules to make attractive interactions with PNIPAM surface causes a decrease in the magnitude of attractive energy with respect to the pure water situation, for basic geometric reasons. Polymer chains collapse to reduce this geometric frustration.


 Please wait while we load your content...
Please wait while we load your content...