Isomerization of the methoxy radical revisited: the impact of water dimers†
Abstract
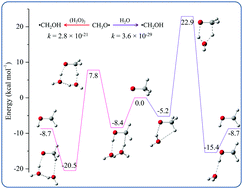
Investigations carried out at MP2 and CCSD(T) levels of theory show that water dimers can compete with formic and sulfuric acids in catalyzing the isomerization of methoxy radicals in the lower troposphere. The rate coefficients for water catalyzed reactions showed opposite temperature dependence to that of acid catalyzed reactions and they were found to play an important role in determining the relative impacts of these catalysts under different atmospheric conditions.

 Please wait while we load your content...
Please wait while we load your content...