Probing the pseudo-1-D ion diffusion in lithium titanium niobate anode for Li-ion battery†
Abstract
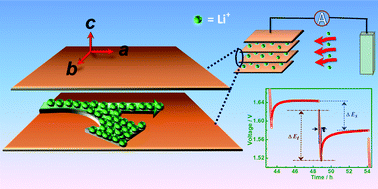
Comprehensive understanding of the charge transport mechanism in the intrinsic structure of an electrode material is essential in accounting for its electrochemical performance. We present here systematic experimental and theoretical investigations of Li+-ion diffusion in a novel layered material, viz. lithium titanium niobate. Lithium titanium niobate (exact composition Li0.55K0.45TiNbO5·1.06H2O) is obtained from sol–gel synthesized potassium titanium niobate (KTiNbO5) by an ion-exchange method. The Li+-ions are inserted and de-inserted preferentially into the galleries between the octahedral layers formed by edge and corner sharing TiO6 and NbO6 octahedral units and the effective chemical diffusion coefficient, is estimated to be 3.8 × 10−11 cm2 s−1 using the galvanostatic intermittent titration technique (GITT). Calculations based on density functional theory (DFT) strongly confirm the anisotropic Li+-ion diffusion in the interlayer galleries and that Li+-ions predominantly diffuse along the crystallographic b-direction. The preferential Li+-ion diffusion along the b-direction is assisted by line-defects, which are observed to be higher in concentration along the b-direction compared to the a- and c-directions, as revealed by high resolution electron microscopy. The Li–Ti niobate can be cycled to low voltages (≈0.2 V) and show stable and satisfactory battery performance over 100 cycles. Due to the possibility of cycling to low voltages, cyclic voltammetry and X-ray photoelectron spectroscopy convincingly reveal the reversibility of Ti3+ ↔ Ti2+ along with Ti4+ ↔ Ti3+ and Nb5+ ↔ Nb4+.


 Please wait while we load your content...
Please wait while we load your content...