Reactivity at the Cu2O(100):Cu–H2O interface: a combined DFT and PES study†
Abstract
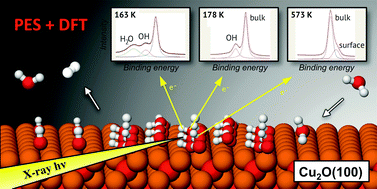
The water–cuprite interface plays an important role in dictating surface related properties. This not only applies to the oxide, but also to metallic copper, which is covered by an oxide film under typical operational conditions. In order to extend the currently scarce knowledge of the details of the water-oxide interplay, water interactions and reactions on a common Cu2O(100):Cu surface have been studied using high-resolution photoelectron spectroscopy (PES) as well as Hubbard U and dispersion corrected density functional theory (PBE-D3+U) calculations up to a bilayer water coverage. The PBE-D3+U results are compared with PBE, PBE-D3 and hybrid HSE06-D3 calculation results. Both computational and experimental results support a thermodynamically favored, and H2O coverage independent, surface OH coverage of 0.25–0.5 ML, which is larger than the previously reported value. The computations indicate that the results are consistent also for ambient temperatures under wet/humid and oxygen lean conditions. In addition, both DFT and PES results indicate that the initial (3,0;1,1) surface reconstruction is lifted upon water adsorption to form an unreconstructed (1 × 1) Cu2O(100) structure.

 Please wait while we load your content...
Please wait while we load your content...