Hydrogen evolution from water using Mo–oxide clusters in the gas phase: DFT modeling of a complete catalytic cycle using a Mo2O4−/Mo2O5− cluster couple
Abstract
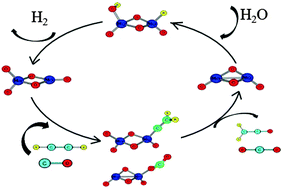
Density functional theory (DFT) calculations using a small metal cluster couple, Mo2O4−/Mo2O5−, are used to model a complete catalytic cycle for H2 production from water. While Mo2O4− is known to readily react with water to form Mo2O5− and release H2, the principal challenge is in reducing Mo2O5− to Mo2O4− to complete the cycle. We investigate the role of several potential sacrificial reagents (ethylene, propylene, CO and acetylene) that can reduce Mo2O5− after the initial oxidation. DFT calculations of the free energy reaction pathways demonstrate the presence of overall kinetically accessible barriers that are below the entrance channel (separated reactants) in the Mo2O4− + H2O reaction (step I) followed by the Mo2O5− + sacrificial reagent reactions (step II). Though the overall reaction is thermodynamically favorable, the first step is highly exothermic while the second step is endothermic. The deepest part of the potential energy surface is a complex of Mo2O5− with the sacrificial reagent. If the energy gained in the first reaction and the succeeding complex formation is not lost due to collisions, the subsequent barriers can be overcome, leading to possible catalytic applications of the Mo2O4−/Mo2O5− cluster couple in H2 production reactions.

 Please wait while we load your content...
Please wait while we load your content...