Molecular modeling of ions at interfaces: exploring similarities to hydrophobic solvation through the lens of induced aqueous interfacial fluctuations†
Abstract
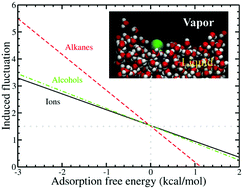
Ion specific effects are ubiquitous in chemistry and biology. While ion specific effects in molecular simulations are generally explained as the result of an interplay between ionic hydration, non-electrostatic potentials and ionic polarizability, this perspective considers an alternative discussion of the coupling of ionic hydration and an interfacial solvent as a contributing component leading to differing interfacial stabilities amongst ions. Interfacially stable ions, characterized as such by minima in free energy profiles, induce larger interfacial fluctuations compared to non-interfacial active species, conferring more covariance entropy approaching the interface. Larger anions, particularly those that are modeled classically with low charge-density, behave as canonical hydrophobic solutes with respect to their solvent-mediated interactions with soft interfaces; whereas smaller anions and cations show no interfacially stable states, nor enhanced interfacial fluctuations. Underlying this phenomenon is the fundamental nature of the hydration shell structure, dynamics, and rigidity around the solutes.
- This article is part of the themed collection: PCCP Perspectives

 Please wait while we load your content...
Please wait while we load your content...