Sorption of 3-hydroxyflavone within channel type zeolites: the effect of confinement on copper(ii) complexation†
Abstract
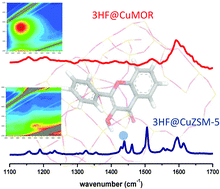
The confinement effect on the complexation process of Cu(II) by 3-hydroxyflavone (3HF) was investigated by studying 3HF incorporation in channel-type copper-containing ZSM-5 and mordenite (MOR) zeolites characterized by different pore diameters. Complementary electronic and vibrational spectroscopy techniques point out two distinct behaviors upon 3HF sorption and subsequent complexation depending on the channel diameter in CuZSM-5 and CuMOR. To determine the influence of the internal environment on the interaction between the copper cation and the guest molecule, and to predict the structure of the complexes formed within the narrow-pore ZSM-5 and in the larger pore mordenite, the vibrational spectra of the complexes were calculated using quantum chemical calculations at the DFT level. From the calculations, it is derived that the Cu(3HF)+ chelate is formed in CuMOR indicating a weak interaction with the pore walls. In contrast, due to high confinement in CuZSM-5, interactions between copper cations and the narrower pore walls are assumed to take place in addition to 3HF metal complexation. To emphasize the fact that zeolites act as a solid solvent, 3HF complexation was also investigated in methanol solution. In such liquid media, a stable complex Cu(3HF)2 of 1 : 2 stoichiometry resulting in a double chelation with the metal cation was found to coexist with a minor species [Cu(3HF)(MeOH)2]+ of 1 : 1 stoichiometry. These two complexes show striking analogy with those observed in CuZSM-5 and CuMOR, respectively. Thus, it appears clearly that zeolites can constitute an ideal tool to control and orientate molecular reactivity for the guest in the isolated state.

 Please wait while we load your content...
Please wait while we load your content...