Structure and electronic properties of bilayer graphene functionalized with half-sandwiched transition metal-cyclopentadienyl complexes†
Abstract
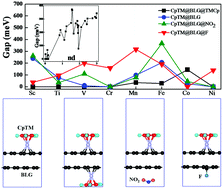
Tuning the electronic and magnetic properties of graphene is a crucial problem in the design of practical on–off electronic devices. Using density functional theory calculations, we explore the electronic and magnetic properties of bilayer graphene functionalized by cyclopentadienyl (Cp = cyclopentadienyl, C5H5) based half-sandwich ligands, CpTM (TM = Sc–Ni). It is found that the adsorption of CpTM ligands can introduce high magnetic moments and open the band gap of bilayer graphene, due to the electron doping as well as the asymmetric charge distribution between two graphene layers. Furthermore, the p–n doping of bilayer graphene by co-binding F/NO2 and CpTM on two external sides of BLG can further widen the band gap up to 366.1 meV. This study proposes an effective way to the modulation of the electronic and magnetic properties of graphene.

 Please wait while we load your content...
Please wait while we load your content...