A unified description of the double perovskite family Sr2MWO6 within a rigid ion model
Abstract
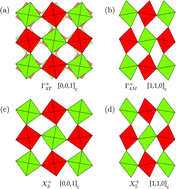
The sequence of phase transitions and structural instabilities of the Sr2MWO6 double perovskites are investigated using a rigid ion model. The parametrization of the short range empirical potential allows the control of the cation sizes by means of independent parameters, and in particular, the effective size of the M cation can be tuned to reproduce the behaviour of the whole family. The coupling of symmetry modes and its role in the stability of the phases are discussed, and molecular dynamics simulations are carried out to determine structural phase transitions as a function of temperature. A satisfactory agreement between experiments and ab initio calculations is obtained for the relevant range of ionic radii and temperatures, indicating that the range of stability of the structures is mainly governed by steric effects.

 Please wait while we load your content...
Please wait while we load your content...