Dynamically slow solid-to-solid phase transition induced by thermal treatment of DimimFeCl4 magnetic ionic liquid†
Abstract
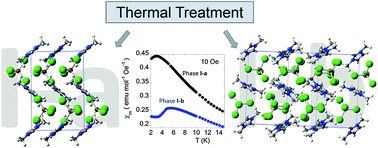
The results reported here represent the first direct experimental observations supporting the existence of a solid-to-solid phase transition induced by thermal treatment in magnetic ionic liquids (MILs). The phase transitions of the solid phases of 1,3-dimethylimidazolium tetrachloroferrate, DimimFeCl4, are closely related to its thermal history. Two series of solid-to-solid phase transitions can be described in this MIL: (i) from room temperature (RT) phase II [space group (s.g.) = P21] to phase I-a [s.g. = P212121] via thermal quenching or via fast cooling at T > 2 K min−1; (ii) from phase I-a to phase I-b [s.g. = P21/c] when the temperature was kept above 180 K for several minutes. The latter involves a slow translational and reorientational dynamical process of both the imidazolium cation and the tetrachloroferrate anion and has been characterized using synchrotron and neutron powder diffraction and DFT (density functional theory) studies. The transition is also related to the modification of the super-exchange pathways of low-temperature phases which show a overall antiferromagnetic behavior. A combination of several experimental methods such as magnetometry, Mössbauer and muon spectroscopy together with polarized and non-polarized neutron powder diffraction has been used in order to characterize the different features observed in these phases.

 Please wait while we load your content...
Please wait while we load your content...