Location of deuterium sites at operating temperature from neutron diffraction of BaIn0.6Ti0.2Yb0.2O2.6−n(OH)2n, an electrolyte for proton-solid oxide fuel cells†
Abstract
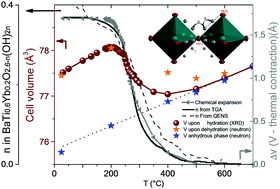
A fundamental understanding of the doping effect on the hydration mechanism and related proton diffusion pathways are keys to the progress of Proton-Solid Oxide Fuel Cell (H+-SOFC) technologies. Here, we elucidate the possible interplay between the crystal structure upon hydration and the conductivity properties in a promising perovskite type H+-SOFC electrolyte, BaIn0.6Yb0.2Ti0.2O2.6−n(OH)2n. Thermal X-ray and neutron diffractions, neutron time-of-flight scattering along with thermal gravimetric analysis reveal the structural features of BaIn0.6Ti0.2Yb0.2O2.6−n(OH)2n at fuel cell operating temperatures. Between 400–600 °C, BaIn0.6Yb0.2Ti0.2O2.6−n(OD)2n (n < 0.042) remains in a disordered perovskite structure with high anisotropies in the form of oblate spheroids for oxygen. At 400 °C, the presence of oxygen and proton static disorder is clearly established. Yet, the insertion of mobile protons in 24k sites does not induce long-range structural distortion while facilitating both inter- and intra-octahedral proton transfers via quasi-linear O–D⋯O bonds, strong hydrogen bonding, and octahedral tilting. This experimental evidence reveals that the co-doping approach on Ba2In2O5 enhances greatly protonic conductivity levels by enabling a continuous proton diffusion pathway through BaIn0.6Yb0.2Ti0.2O2.6−n(OH)2n. These new insights into the doping effect on the proton-transfer mechanism offer new perspectives for the development of H+-SOFC electrolyte materials.

 Please wait while we load your content...
Please wait while we load your content...