Chemical vapor deposition of MoS2 layers from Mo–S–C–O–H system: thermodynamic modeling and validation†
Abstract
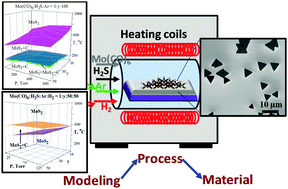
A detailed thermodynamic analysis of the solid and gas phases of the Mo–S–C–O–H system used for large area chemical vapor deposition (CVD) of MoS2 is presented and compared with experimental results. Given the multivariable nature of the problem, excellent agreement is observed. Deviations, observed from thermodynamic predictions, mainly at low temperatures and high flow rates have been highlighted and discussed. CVD phase diagrams which predict parameter windows in which pure MoS2 can be synthesized have been provided for important gas phase chemistries. Pure H2 as a carrier gas is shown to facilitate the largest contamination free process window. CO presence is shown to significantly reduce the nucleation rate and enable large island sizes but at the cost of carbon contamination. Oxygen leaks are shown to result in sulphur contamination. The absence of H2S during cooling is shown to yield Mo due to the reduction of MoS2 by hydrogen. Oxidation of Mo causes oxide contamination.

 Please wait while we load your content...
Please wait while we load your content...