Positron annihilation and nuclear magnetic resonance study of the phase behavior of water confined in mesopores at different levels of hydration†
Abstract
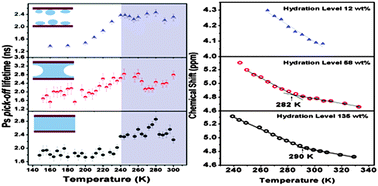
We investigated the molecular origin of the phase behavior of water confined in MCM 41 mesopores at different levels of hydration using positron annihilation spectroscopic and nuclear magnetic resonance techniques. The level of hydration influenced the phase behavior of the nanoconfined water. Two transitions above and below the bulk freezing temperature were observed depending on the level of hydration. At the highest level of hydration, nucleation seemed to predominate over the effect of confinement, leading to the complete freezing of water, whereas disrupted H-bonding dominated at the lowest level of hydration, leading to the disappearance of the transitions. A transition at c. T = 188 K (close to the reported glass transition temperature of interface-affected water) was observed at intermediate hydration level. This study suggests that the H-bonding network within nanoconfined water, which can be tampered by the degree of hydration, is the key factor responsible for the phase behavior of supercooled water. This study on the phase behavior and associated transitions of nanoconfined water has implications for nanofluidics and drug-delivery systems, in addition to understanding the fundamentals of water in confinement.

 Please wait while we load your content...
Please wait while we load your content...