Electrochemical activity and high ionic conductivity of lithium copper pyroborate Li6CuB4O10†
Abstract
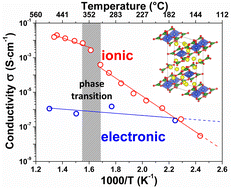
In the search for new cathode materials for Li-ion batteries, borate (BO33−) based compounds have gained much interest during the last two decades due to the low molecular weight of the borate polyanions which leads to active materials with increased theoretical capacities. In this context we herein report the electrochemical activity versus lithium and the ionic conductivity of a diborate or pyroborate B2O54− based compound, Li6CuB4O10. By combining various electrochemical techniques with in situ X-ray diffraction, we show that this material can reversibly insert/deinsert limited amounts of lithium (∼0.3 Li+) in a potential window ranging from 2.5 to 4.5 V vs. Li+/Li0. We demonstrate, via electron paramagnetic resonance (EPR), that such an electrochemical activity centered near 4.25 V vs. Li+/Li0 is associated with the Cu3+/Cu2+ redox couple, confirmed by density functional theory (DFT) calculations. Another specificity of this compound lies in its different electrochemical behavior when cycled down to 1 V vs. Li+/Li0 which leads to the extrusion of elemental copper via a conversion type reaction as deduced by transmission electron microscopy (TEM). Lastly, we probe the ionic conductivity by means of AC and DC impedance measurements as a function of temperature and show that Li6CuB4O10 undergoes a reversible structural transition around 350 °C, leading to a surprisingly high ionic conductivity of ∼1.4 mS cm−1 at 500 °C.


 Please wait while we load your content...
Please wait while we load your content...