The removal of disulfide bonds in amylin oligomers leads to the conformational change of the ‘native’ amylin oligomers†
Abstract
The α-helical structure of the N-terminus of the ‘native’ amylin Lys1–Cys7 consists of a disulfide bond between Cys2 and Cys7. The ‘native’ amylin oligomers demonstrate polymorphic states. Removal of the disulfide bonds in the ‘native’ amylin oligomers decreases the polymorphism and induces the formation of longer stable cross-β strands in the N-termini.
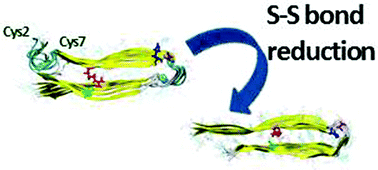

 Please wait while we load your content...
Please wait while we load your content...