In situ pore-forming alginate hydrogel beads loaded with in situ formed nano-silver and their catalytic activity†
Abstract
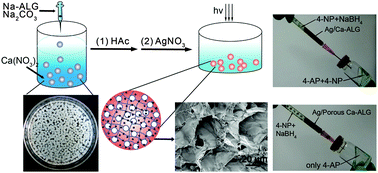
An aqueous mixture of sodium carbonate (Na2CO3) and sodium alginate (Na-ALG) was added dropwise into an aqueous solution of Ca(NO3)2, leading to the formation of calcium alginate (Ca-ALG) hydrogel beads. Meanwhile Na2CO3 as a pore-forming precursor was transformed in situ into CaCO3 nanoparticles (CaCO3 NPs). SEM images show that CaCO3 NPs aggregates with a size of ∼10 μm were uniformly distributed in the Ca-ALG hydrogel beads. After subsequent erosion using acetic acid, Ca-ALG hydrogel beads with a uniform microporous structure were obtained. The porosity and specific surface area of such in situ pore-formed hydrogel beads are 16 and 14 times higher than those of the beads prepared in the absence of Na2CO3. Additionally, their porous structure can be modulated by varying the amount of Na2CO3. The obtained porous Ca-ALG hydrogel beads were further immersed into an aqueous solution of AgNO3. Under UV irradiation, the Ag+ ions adsorbed in the Ca-ALG were in situ reduced to Ag nanoparticles (Ag NPs). SEM and TEM images show that Ag NPs with a size of ∼10 nm were uniformly distributed in the matrix of the hydrogel beads. The loading amount of Ag in the beads can also be modulated by varying the amount of Na2CO3. Furthermore, the resultant Ca-ALG beads loaded with Ag NPs (Ag/Ca-ALG) were used as catalysts. Their catalytic activity was evaluated by using the reduction reaction of 4-nitrophenol as a model reaction. The rate constant of the reaction in the presence of dry porous Ag/Ca-ALG beads was found to be 36 times higher than that in the presence of beads prepared in the absence of Na2CO3. Such a high catalytic efficiency can be attributed to their porous structure and consequent high Ag-loading capacity.

 Please wait while we load your content...
Please wait while we load your content...