Asn47 and Phe114 modulate the inner sphere reorganization energies of type zero copper proteins†
Abstract
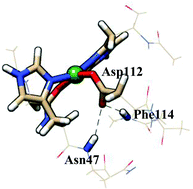
The geometric structures and electron transfer properties of type 1 Cu proteins are reasonably understood at the molecular level (E. I. Solomon and R. G. Hadt, Coord. Chem. Rev., 2011, 255, 774–789, J. J. Warren, K. M. Lancaster, J. H. Richards and H. B. Gray, J. Inorg. Biochem., 2012, 115, 119–126). Much understanding of type 1 copper electron transfer reactivity has come from site directed mutagenesis studies. For example, artificial “type zero” Cu-centres constructed in cupredoxin–azurin have showcased the capacity of outer-sphere hydrogen bonding networks to enhance Cu II/I electron transfer reactivity. In this paper, we have elaborated on earlier kinetics and electronic structural studies of type zero Cu by calculating the inner sphere reorganization energies of type 1, type 2, and type zero Cu proteins using density functional theory (DFT). Although the choice of density functionals for copper systems is not straightforward, we have benchmarked the density functionals against the recently reported ESI-PES data for two synthetic copper models (S. Niu, D.-L. Huang, P. D. Dau, H.-T. Liu, L.-S. Wang and T. J. Ichiye, Chem. Theory Comput., 2014, 10, 1283). For the Cu proteins, our calculations predict that changes in the coordination number upon metal reduction lead to large inner sphere reorganization energies for type 2 Cu sites, whereas retention in the coordination number is observed for type zero Cu sites. These variations in the coordination number are modulated by the outer-sphere coordinating residues Asn47 and Phe114, which are involved in hydrogen bonding with the Asp112 side chain.

 Please wait while we load your content...
Please wait while we load your content...