On the nature of bonding in binary Be2O2 and Si2O2 clusters: rhombic four-center four-electron π and σ bonds
Abstract
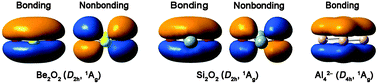
The structural and electronic properties and chemical bonding of binary Be2O2 and Si2O2 clusters have been studied using quantum chemical calculations at the B3LYP level. For the Be2O2 cluster, the potential energy surface is probed by unbiased structural searches and the global-minimum structure was established using the B3LYP calculations, complemented by PBE0 and single-point CCSD(T) calculations for top isomers. The perfectly planar D2h Be2O2 (1Ag) global minimum is well defined, being at least 3.64 eV lower in energy than alternative structures at the CCSD(T)//B3LYP/aug-cc-pVTZ level. Chemical bonding analyses show that D2h Be2O2 and Si2O2 clusters possess the rhombic four-center four-electron (4c–4e) π bond, that is, the o-bond, a conception derived from electron-deficient boron oxide clusters lately. Furthermore, the Be2O2 and Si2O2 clusters also exhibit rhombic 4c–4e σ bonds, both for the radial and tangential σ frameworks (σr and σt). The σt framework is classified as an o-bond only formally, due to the secondary contribution from the Be/Si s component. The three-fold (π, σr, and σt) o-bonds in Be2O2 and Si2O2 are considered to resemble the three-fold aromaticity in all-metal Al42− dianions. A 4c–4e o-bond makes use of four O 2p electrons, which would otherwise be two lone-pairs, for a delocalized and completely bonding orbital, as well as a residual nonbonding orbital. Three-fold o-bonds thus greatly stabilize the binary Be2O2 and Si2O2 clusters. We anticipate that the bonding concept should be applicable to additional molecular systems, including those with larger heterocyclic rings.

 Please wait while we load your content...
Please wait while we load your content...