Anisotropic Li intercalation in a LixFePO4 nano-particle: a spectral smoothed boundary phase-field model
Abstract
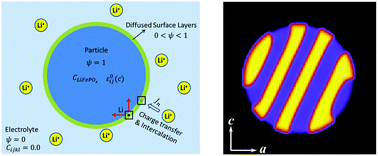
A spectral smoothed boundary phase-field model is implemented to study lithium (Li) intercalation in a LixFePO4 nano-particle immersed in a Li+ rich electrolyte. It takes into account different physical processes on the particle surface, such as heterogeneous nucleation, Li flux and stress-free boundary conditions. We show the nucleation and growth of plate-like Li-rich crystallites along the (010) plane due to the high Li mobility along [001]. Since such plate-like crystallites, which are nucleated from (001) surfaces, align their phase boundaries along the (101) habit planes, a LixFePO4 nano-particle with prominent (010) and (001) surface facets and the longest axis length along [100] is proposed to exhibit great mechanical stability.

 Please wait while we load your content...
Please wait while we load your content...