Improving dipolar recoupling for site-specific structural and dynamics studies in biosolids NMR: windowed RN-symmetry sequences†
Abstract
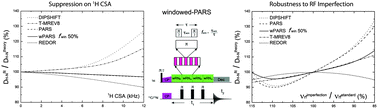
Experimental characterization of one-bond heteronuclear dipolar couplings is essential for structural and dynamics characterization of molecules by solid-state NMR. Accurate measurement of heteronuclear dipolar tensor parameters in magic-angle spinning NMR requires that the recoupling sequences efficiently reintroduce the desired heteronuclear dipolar coupling term, fully suppress other interactions (such as chemical shift anisotropy and homonuclear dipolar couplings), and be insensitive to experimental imperfections, such as radio frequency (rf) field mismatch. In this study, we demonstrate that the introduction of window delays into the basic elements of a phase-alternating R-symmetry (PARS) sequence results in a greatly improved protocol, termed windowed PARS (wPARS), which yields clean dipolar lineshapes that are unaffected by other spin interactions and are largely insensitive to experimental imperfections. Higher dipolar scaling factors can be attained in this technique with respect to PARS, which is particularly useful for the measurement of relatively small dipolar couplings. The advantages of wPARS are verified experimentally on model molecules N-acetyl-valine (NAV) and a tripeptide Met-Leu-Phe (MLF). The incorporation of wPARS into 3D heteronuclear or homonuclear correlation experiments permits accurate site-specific determination of dipolar tensors in proteins, as demonstrated on dynein light chain 8 (LC8). Through 3D wPARS recoupling based spectroscopy we have determined both backbone and side chain dipolar tensors in LC8 in a residue-resolved manner. We discuss these in the context of conformational dynamics of LC8. We have addressed the effect of paramagnetic relaxant Cu(II)-EDTA doping on the dipolar coupling parameters in LC8 and observed no significant differences with respect to the neat sample permitting fast data collection. Our results indicate that wPARS is advantageous with respect to the windowless version of the sequence and is applicable to a broad range of systems including but not limited to biomolecules.

 Please wait while we load your content...
Please wait while we load your content...