Structural transformation during Li/Na insertion and theoretical cyclic voltammetry of the δ-NH4V4O10 electrode: a first-principles study†
Abstract
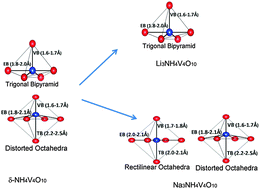
A double layer δ-NH4V4O10, due to its high energy storage capacity and excellent rate capability, is a very promising cathode material for Li-ion and Na-ion batteries for large-scale renewable energy storage in transportation and smart grids. While it possesses better stability, and higher ionic and electronic conductivity than the most widely explored V2O5, the mechanisms of its cyclability are yet to be understood. Here, we present a theoretical cyclic voltammetry as a tool based on first-principles calculations, and uncover structural transformations that occur during Li+/Na+ insertion (x) into (Lix/Nax)NH4V4O10. Structural distortions associated with single-phase and multi-phase structural changes during the insertion of Li+/Na+, identified through the analysis of voltage profile and theoretical cyclic voltammetry are in agreement with the reported experimental electrochemical measurements on δ-NH4V4O10. We obtain an insight into its electronic structure with a lower band gap that is responsible for the high rate capability of (Lix/Nax) δ-NH4V4O10. The scheme of theoretical cyclic voltammetry presented here will be useful for addressing issues of cyclability and energy rate in other electrode materials.

 Please wait while we load your content...
Please wait while we load your content...