A general view on the reactivity of the oxygen-functionalized graphene basal plane
Abstract
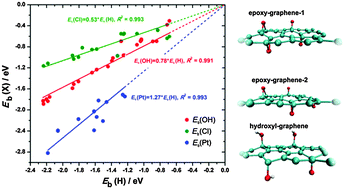
In this contribution we inspect the adsorption of H, OH, Cl and Pt on oxidized graphene using DFT calculations. The introduction of epoxy and hydroxyl groups on the graphene basal plane significantly alters its chemisorption properties, which can be attributed to the deformation of the basal plane and the type and distribution of these groups. We show that a general scaling relation exists between the hydrogen binding energies and the binding energies of other investigated adsorbates, which allows for a simple probing of the reactivity of oxidized graphene with only one adsorbate. The electronic states of carbon atoms located within the 2 eV interval below the Fermi level are found to be responsible for the interaction of the basal plane with the chosen adsorbates. The number of electronic states situated in this energy interval is shown to correlate with hydrogen binding energies.

 Please wait while we load your content...
Please wait while we load your content...