Physical state of cellulose in BmimCl: dependence of molar mass on viscoelasticity and sol-gel transition†
Abstract
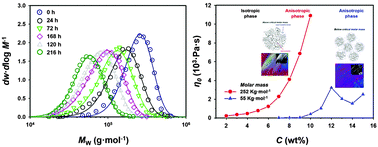
In this work, we investigated the correlation between the molar mass and the rheological properties of cellulose/1-butyl-3-methylimidazolium chloride (BmimCl) solutions, and provided the depolymerization kinetics of cellulose in BmimCl. Gel permeation chromatography was used to track the change in molar mass and kinetics as a function of the dissolution time. The molar mass of cellulose in BmimCl decreased significantly as the dissolution time increased, following a zeroth order rate law. The decrease of inter-chain friction induced by depolymerization resulted in a lower viscosity, shorter relaxation time, and lower activation energy. The activation energies for flow were distinctly different above and below the critical molar mass, which indicates that the relaxation mechanisms were not identical above and below the critical molar mass. The transition behavior of liquid crystalline phase also changed at the critical molar mass, which strongly demonstrated the effect of chain length on the formation of cholesteric phase. The exponents of Mark–Houwink–Sakurada and the radius of gyration showed that cellulose in BmimCl existed as a Gaussian chain in a theta solvent.

 Please wait while we load your content...
Please wait while we load your content...