Structural motifs of 2-(2-fluoro-phenyl)-ethylamine conformers†
Abstract
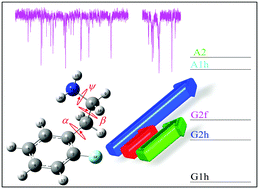
Vibronic and vibrational spectra of 2-(2-fluoro-phenyl)-ethylamine (2-FPEA) conformers were measured in a molecular beam by resonant two-photon ionization (R2PI), ultraviolet–ultraviolet hole burning (UV–UV HB) spectroscopy, and ionization-loss stimulated Raman spectroscopy (ILSRS). The measured ILSR spectral signatures in the survey spectra of the amino group region and in the broad spectral range revealed the presence of five different conformers, which were confirmed by the HB spectra. The determination of the structures of the conformers of 2-FPEA was assisted by quantum chemical calculations of the torsional potential energy surface and of the scaled harmonic Raman spectra. Comparison of the measured ILSR spectra with the calculated Raman spectra allowed us to identify one gauche structure with the ethylamino side chain folded toward the fluorine atom, two gauche structures with the ethylamino side chain folded to the opposite side and two anti conformers with extended tails. The effect of fluorination on the spectra and on the stability and structures of these species is discussed.

 Please wait while we load your content...
Please wait while we load your content...