Liquid–liquid equilibria of binary mixtures of a lipidic ionic liquid with hydrocarbons
Abstract
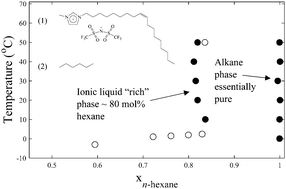
Although structurally diverse, many ionic liquids (ILs) are polar in nature due to the strong coulombic forces inherent in ionic compounds. However, the overall polarity of the IL can be tuned by incorporating significant nonpolar content into one or more of the constituent ions. In this work, the binary liquid–liquid equilibria of one such IL, 1-methyl-3-(Z-octadec-9-enyl)imidazolium bistriflimide, with several hydrocarbons (n-hexane, n-octane, n-decane, cyclohexane, methylcyclohexane, 1-octene) is measured over the temperature range 0–70 °C at ambient pressure using a combination of cloud point and gravimetric techniques. The phase behavior of the systems are similar in that they exhibit two phases: one that is 60–90 mole% hydrocarbon and a second phase that is nearly pure hydrocarbon. Each phase exhibits a weak dependence of composition on temperature (steep curve) above ∼10 °C, likely due to swelling and restructuring of the nonpolar nano-domains of the IL being limited by energetically unfavorable restructuring in the polar nano-domains. The solubility of the n-alkanes decreases with increasing size (molar volume), a trend that continues for the cyclic alkanes, for which upper critical solution temperatures are observed below 70 °C. 1-Octene is found to be more soluble than n-octane, attributable to a combination of its lower molar volume and slightly higher polarity. The COSMO-RS model is used to predict the T-x′-x′′ diagrams and gives good qualitative agreement of the observed trends. This work presents the highest known solubility of n-alkanes in an IL to date and tuning the structure of the ionic liquid to maximize the size/shape trends observed may provide the basis for enhanced separations of nonpolar species.

 Please wait while we load your content...
Please wait while we load your content...