Chemical and photochemical properties of chloroharmine derivatives in aqueous solutions†
Abstract
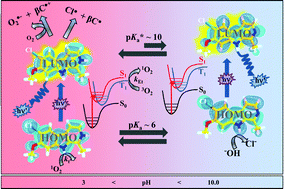
Thermal and photochemical stability (ΦR), room temperature UV-vis absorption and fluorescence spectra, fluorescence quantum yields (ΦF) and lifetimes (τF), quantum yields of hydrogen peroxide (ΦH2O2) and singlet oxygen (ΦΔ) production, and triplet lifetimes (τT) have been obtained for the neutral and protonated forms of 6-chloroharmine, 8-chloroharmine and 6,8-dichloroharmine, in aqueous media. When it was possible, the effect of pH and oxygen concentration was evaluated. The nature of electronic transitions of protonated and neutral species of the three investigated chloroharmines was established using Time-Dependent Density Functional Theory (TD-DFT) calculations. The impact of all the foregoing observations on the biological role of the studied compounds is discussed.

 Please wait while we load your content...
Please wait while we load your content...