Is the boundary layer of an ionic liquid equally lubricating at higher temperature?†
Abstract
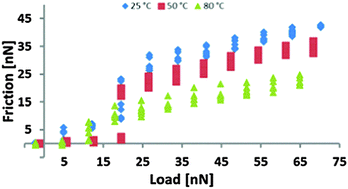
Atomic force microscopy has been used to study the effect of temperature on normal forces and friction for the room temperature ionic liquid (IL) ethylammonium nitrate (EAN), confined between mica and a silica colloid probe at 25 °C, 50 °C, and 80 °C. Force curves revealed a strong fluid dynamic influence at room temperature, which was greatly reduced at elevated temperatures due to the reduced liquid viscosity. A fluid dynamic analysis reveals that bulk viscosity is manifested at large separation but that EAN displays a nonzero slip, indicating a region of different viscosity near the surface. At high temperatures, the reduction in fluid dynamic force reveals step-like force curves, similar to those found at room temperature using much lower scan rates. The ionic liquid boundary layer remains adsorbed to the solid surface even at high temperature, which provides a mechanism for lubrication when fluid dynamic lubrication is strongly reduced. The friction data reveals a decrease in absolute friction force with increasing temperature, which is associated with increased thermal motion and reduced viscosity of the near surface layers but, consistent with the normal force data, boundary layer lubrication was unaffected. The implications for ILs as lubricants are discussed in terms of the behaviour of this well characterised system.

 Please wait while we load your content...
Please wait while we load your content...