Proton triggered emission and selective sensing of picric acid by the fluorescent aggregates of 6,7-dimethyl-2,3-bis-(2-pyridyl)-quinoxaline
Abstract
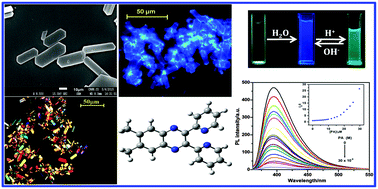
A heteroatom containing organic fluorophore 6,7-dimethyl-2,3-bis-(2-pyridyl)-quinoxaline (BPQ) is weakly emissive in solution but its emission properties are highly enhanced in the aggregated state due to the restriction of intramolecular rotation (RIR) and large amplitude vibrational modes, demonstrating the phenomenon, aggregation induced emission enhancement (AIEE). It has strong proton capture capability, allowing reversible fluorescence switching in basic and acidic medium and the emission color changes from blue to green in the aggregated state through protonation. It has been explained as a competition between intramolecular charge transfers (ICTs) and the AIEE phenomena at a lower pH range (pH ∼1–4). Such behavior enables it as a fluorescent pH sensor for detection in acidic and basic medium. Morphologies of the particles are characterized using optical and field emission scanning electron microscopic (FESEM) studies. The turn off fluorescence properties of aggregated BPQ have been utilized for the selective detection of picric acid and the fluorescence quenching is explained due to ground state complexation with a strong quenching constant, 7.81 × 104 M−1.

 Please wait while we load your content...
Please wait while we load your content...