Using the C–O stretch to unravel the nature of hydrogen bonding in low-temperature solid methanol–water condensates
Abstract
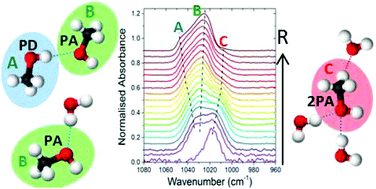
Transmission infrared spectroscopy has been used in a systematic laboratory study to investigate hydrogen bonding in binary mixtures of CH3OH and H2O, vapour deposited at 30 K, as a function of CH3OH/H2O mixing ratio, R. Strong intermolecular interactions are evident between CH3OH and H2O with infrared band profiles of the binary ices differing from that of the pure components and changing significantly with R. Consistent evidence from the O–H and C–H band profiles and detailed analysis of the C–O stretch band reveal two different hydrogen bonding structural regimes below and above R = 0.6–0.7. The vapour deposited solid mixtures were found to exhibit behaviour similar to that of liquids with evidence of inhomogeneity and higher coordination number of hydrogen bonds that are concentration dependent. The C–O stretch band is found to consist of three components around 1039 cm−1 (‘blue’), 1027 cm−1 (‘middle’) and 1011 cm−1 (‘red’). The ‘blue’ and ‘middle’ components corresponding to environments with CH3OH dominating as a proton donor (PD) and proton acceptor (PA) respectively reveal preferential bonding of CH3OH as a PA and H2O as a PD in the mixtures. The ‘red’ component is only present in the presence of H2O and has been assigned to the involvement of both lone pairs of electrons on the oxygen atom of CH3OH as a PA to two PD H2O atoms. Cooperative effects are evident with concurrent blue-shifts in the C–H stretching modes of CH3OH below R = 0.6 indicating CH3 group participation in hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...