Modulation of ultrafast photoinduced electron transfer in H-bonding environment: PET from aniline to coumarin 153 in the presence of an inert co-solvent cyclohexane†
Abstract
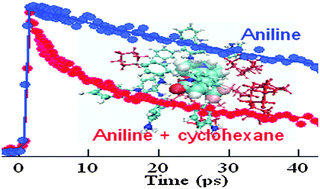
Despite intensive research, the role of the H-bonding environment on ultrafast PET remains illusive. For example, coumarin 153 (C153) undergoes ultrafast photoinduced electron transfer (PET) in electron-donating solvents, in both aniline (AN) and N,N-dimethylaniline (DMA), despite their very different H-bonding abilities. Thus, donor–acceptor (AN–C153) H-bonding may have only a minor role in PET (Yoshihara and co-workers, J. Phys. Chem. A, 1998, 102, 3089). However, donor–acceptor H-bonding may be somehow less effective in the neat H-bonding environment but could become dominant in the presence of an inert solvent (Phys. Chem. Chem. Phys., 2014, 16, 6159). We successfully applied and tested the proposal here. The nature of PET modulation of C153 in the presence of a passive component cyclohexane is found to be very different for aniline and DMA. Upon addition of cyclohexane to DMA, the PET process gradually becomes retarded but in the case of AN, the PET rate was indeed found to be accelerated at some intermediate composition (mole fraction of aniline, XAN ∼ 0.74) compared to that of neat aniline. It is intuitive that cyclohexane may replace some of the donors (AN or DMA) from the vicinity of the acceptor and, thus, should disfavour PET. However, in the hydrogen bonding environment using molecular dynamics simulation, for the first time, we show that the average number of aniline molecules orienting their N–H group in the proximity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of C153 is actually higher at the intermediate mole fraction (0.74) of aniline in a mixture rather than in neat aniline. This small but finite excess of C153–AN H-bonding already present in the ground state may possibly account for the anomalous effect. The TD-DFT calculations presented here showed that the intermolecular H-bonding between C153 and AN strengthens from 21.1 kJ mol−1 in the ground state to 33.0 kJ mol−1 in the excited state and, consequently, H-bonding may assist PET according to the Zhao and Han model. Thus, we not only justified both the theoretical prediction (efficient H-bond assisted PET within the C153–AN pair) and experimental observation (minor H-bond assisted PET in neat solvent) but also established our previous hypothesis that an inert co-solvent can enhance the effect of H-bonding from molecular insights.
O group of C153 is actually higher at the intermediate mole fraction (0.74) of aniline in a mixture rather than in neat aniline. This small but finite excess of C153–AN H-bonding already present in the ground state may possibly account for the anomalous effect. The TD-DFT calculations presented here showed that the intermolecular H-bonding between C153 and AN strengthens from 21.1 kJ mol−1 in the ground state to 33.0 kJ mol−1 in the excited state and, consequently, H-bonding may assist PET according to the Zhao and Han model. Thus, we not only justified both the theoretical prediction (efficient H-bond assisted PET within the C153–AN pair) and experimental observation (minor H-bond assisted PET in neat solvent) but also established our previous hypothesis that an inert co-solvent can enhance the effect of H-bonding from molecular insights.

 Please wait while we load your content...
Please wait while we load your content...