Stabilization of Al(iii) solutions by complexation with cacodylic acid: speciation and binding features†
Abstract
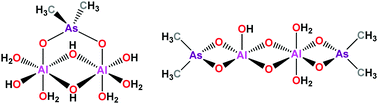
Aluminium ions are believed to play a role in a number of neurological and skeletal disorders in the human body. The study of the biological processes and molecular mechanisms that underlie these pathological disorders is rendered a difficult task due to the wide variety of complex species that result from the hydrolysis of Al3+ ions. In addition, this ion displays a pronounced tendency to precipitate as a hydroxide, so certain complexing agents should be envisaged to stabilize Al(III) solutions in near physiological conditions. In this work, we show that the common buffer cacodylic acid (dimethylarsinic acid, HCac) interacts with Al(III) to give stable complexes, even at pH 7. After preliminary analyses of the speciation of the metal ion and also of the ligand, a systematic study of the formation of different Al/Cac complexes at different pH values has been conducted. UV-Vis titrations, mass spectrometry NMR measurements and DTF calculations were performed to enlighten the details of the speciation and stoichiometry of Al/Cac complexes. The results altogether show that Al/Cac dimer complexes prevail, but monomer and trimer forms are also present. Interestingly, it was found that cacodylate promotes the formation of such relatively simple complexes, even under conditions where the polymeric form, Al13O4(OH)247+, should predominate. The results obtained can help to shed some light into the reactivity of aluminium ions in biological environments.

 Please wait while we load your content...
Please wait while we load your content...