When do defectless alkanethiol SAMs in ionic liquids become penetrable? A molecular dynamics study†
Abstract
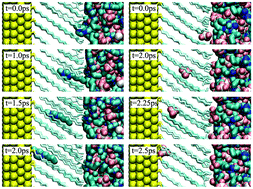
Molecular dynamics simulations were performed to address the permeability of defectless alkanethiol self-assembled monolayers (SAMs) on charged and uncharged Au(111) surfaces in 1-butyl-3-methylimidazolium ([bmim][BF4]) room-temperature ionic liquid (IL). We demonstrate that ionic permeation into the monolayer does not start until a critical surface charge density value is attained (both for positive and negative surface charges). The free energy barrier for the permeation of IL components is shown to include nearly equal contributions from ion desolvation and the channel formation in the dense monolayer. Long chain alkanethiols (hexadecanethiol SC16H33) exhibit superior barrier properties as compared with short chain alkanethiols (hexanethiol SC6H13) due to the dense packing of alkanethiol chains in highly ordered zigzag conformation oriented at the same tilt angle. Computed critical charge densities correspond to the electrode potential values beyond the limits of the monolayer stability, which might indicate the impermeability of the defectless monolayer towards the IL components. Experimental findings on increased interfacial capacitance are interpreted, therefore as some manifestation of the monolayer defectiveness occurring in real electrochemical systems. The potential of the mean force is constructed for a typical redox probe ferrocene/ferrocenium (Fc/Fc+) as well, to investigate a possible permeation of the solute from the IL into the SC6H13 monolayer.

 Please wait while we load your content...
Please wait while we load your content...