Efficient intersystem crossing using singly halogenated carbomethoxyphenyl porphyrins measured using delayed fluorescence, chemical quenching, and singlet oxygen emission
Abstract
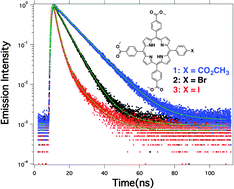
Sensitizers with high triplet quantum yields are useful for generating photovoltaics, photocatalysts and photodynamic therapy agents with increased efficiency. In this study, the heavy atom effect was used to optimize the triplet and singlet oxygen quantum yields of 5,10,15,20-tetrakis(4-carbomethoxyphenyl)porphyrin (1-TCM4PP). The triplet quantum yields, determined using delayed fluorescence, was calculated as 0.35 for 1-TCM4PP, 0.75 for 5,10,15-tris(4-carbomethoxyphenyl)-20-(4-bromophenyl)porphyrin (2-TBCM3PP) and 0.88 for 5,10,15-tris(4-carbomethoxyphenyl)-20-(4-iodophenyl)porphyrin (3-TCM3IPP). Chemical quenching of 1,3-diphenylisobenzofuran and singlet oxygen emission studies rendered an average singlet oxygen quantum yield of 0.51, 0.75, and 0.90 for TCM4PP, TBCM3PP and TCM3IPP respectively. These photophysical properties indicate that a single halogen atom is capable of transforming TCM4PP into a sensitizer with strong triplet character. This is useful for generating singlet oxygen for photodynamic therapy, creating a long lasting reactive species for catalysis and for extending diffusion lengths in photovoltaic applications while retaining three molecular modification points for further functionalization.

 Please wait while we load your content...
Please wait while we load your content...