A global full-dimensional potential energy surface and quasiclassical trajectory study of the O(1D) + CH4 multichannel reaction
Abstract
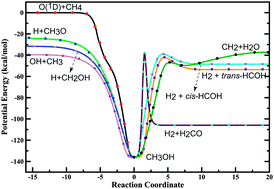
We report a new global, full-dimensional ground-state potential energy surface (PES) of the O(1D) + CH4 multichannel reaction, based on high-level ab initio calculations and fitting procedures. The PES is a permutationally invariant fit to roughly 340 000 electronic energies calculated by the MRCI + Q/aug-cc-pVTZ level of theory. Extensive quasiclassical trajectory calculations were carried out on the new PES at the collision energy of relevance to the previously universal crossed molecular beam experiments. The product branching ratios, translational energy distributions and angular distributions of OH + CH3, H + CH2OH/CH3O and H2 + HCOH/H2CO product channels were calculated and compared with the available experimental results. Very good agreement between theory and experiment has been achieved. The O(1D) + CH4 reaction mainly proceeds through the CH3OH intermediate via a trapped abstraction mechanism, starting with the abstraction of the hydrogen atom, rather than the direct insertion pathway with the O(1D) atom directly inserting into the C–H bond of CH4. The process with a very short lifetime behaves like an abstraction reaction, producing a pronounced forward scattering peak as found in the OH + CH3 channel, while the process with a relatively long lifetime produces reaction products with nearly forward and backward scattering symmetry, similar to an insertion reaction, as found in other reaction channels.

 Please wait while we load your content...
Please wait while we load your content...