Quantum mechanical study of the β- and δ-lyase reactions during the base excision repair process: application to FPG†
Abstract
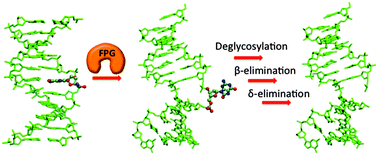
Bacterial FPG (or MutM) is a bifunctional DNA glycosylase that is primarily responsible for excising 8-oxoguanine (OG) from the genome by cleaving the glycosidic bond and the DNA backbone at the 3′- and 5′-phosphates of the damaged nucleoside. In the present work, quantum mechanical methods (SMD-M06-2X/6-311+G(2df,2p)//IEF-PCM-B3LYP/6-31G(d)) and a ring-opened Schiff base model that includes both the 3′- and 5′-phosphate groups are used to investigate the β- and δ-elimination reactions facilitated by FPG. Both the β- and δ-elimination reactions are shown to proceed through an E1cB mechanism that involves proton abstraction prior to the phosphate–ribose bond cleavage. Since transition states for the phosphate elimination reactions could not be characterized in the absence of leaving group protonation, our work confirms that the phosphate elimination reactions require protonation by a residue in the FPG active site, and can likely be further activated by additional active-site interactions. Furthermore, our model suggests that 5′-PO4 activation may proceed through a nearly isoenergetic direct (intramolecular) proton transfer involving the O4′ proton of the deoxyribose of the damaged nucleoside. Regardless, our model predicts that both 3′- and 5′-phosphate protonation and elimination steps occur in a concerted reaction. Most importantly, our calculated barriers for the phosphate cleavage reactions reveal inherent differences between the β- and δ-elimination steps. Indeed, our calculations provide a plausible explanation for why the δ-elimination rather than the β-elimination is the rate-determining step in the BER facilitated by FPG, and why some bifunctional glycosylases (including the human counterpart, hOgg1) lack δ-lyase activity. Together, the new mechanistic features revealed by our work can be used in future large-scale modeling of the DNA–protein system to unveil the roles of key active sites residues in these relatively unexplored BER steps.

 Please wait while we load your content...
Please wait while we load your content...