Computational insights into the inhibition and destabilization of morin on the oligomer of full-length human islet amyloid polypeptide
Abstract
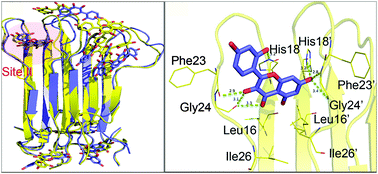
The aggregation of human islet amyloid polypeptide (hIAPP) is closely related with the occurrence of type 2 diabetes (T2D). Natural flavonoid morin was confirmed to not only inhibit the amyloid formation of hIAPP, but disaggregate its preformed amyloid fibrils. In this study, with the goal of elucidating the molecular mechanism of inhibition and destabilization of morin on the full-length hIAPP1–37 oligomer, molecular dynamics simulations were performed for hIAPP1–37 pentamer in the presence and absence of morin. The obtained results show that during the protein–inhibitor interaction, morin can notably alter the structural properties of hIAPP1–37 pentamer, such as morphology, solvent accessible surface area and secondary structure. Moreover, we identified three possible binding sites of morin on hIAPP, all of which located near the amyloidogenic region of this protein. From the binding free energy calculations, we found that Site II was the most possible one. Further conformational analysis together with energy decomposition showed that the residues His18, Phe23 and Ile26 play a key role in the binding with morin by hydrogen bond, π–π and hydrophobic interactions. The proposal of the theoretical mechanism of morin against hIAPP aggregation will provide valuable information for the development of new drugs to inhibit hIAPP aggregation.

 Please wait while we load your content...
Please wait while we load your content...