A DFT study of adsorption of imidazole, triazole, and tetrazole on oxidized copper surfaces: Cu2O(111) and Cu2O(111)-w/o-CuCUS†
Abstract
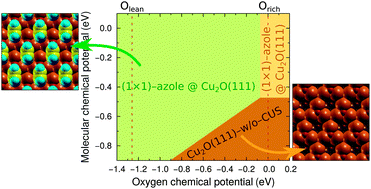
Azoles and their derivatives are known for their corrosion inhibition ability for copper. For this reason the bonding of imidazole, triazole, and tetrazole—used as archetypal models of azole corrosion inhibitors—to Cu2O(111) and Cu2O(111)-w/o-CuCUS was characterized using density functional theory (DFT) calculations. The former surface contains coordinatively-saturated (CSA) and coordinatively-unsaturated (CUS) Cu sites, whereas the latter lacks the CUS sites. We find that the molecules preferentially bond with a single unsaturated N atom to a surface Cu ion and concomitantly form a hydrogen bond with the surface O ion. They adsorb rather strongly at CUS sites with an adsorption energy of about −1.6 eV (as calculated with the PBE functional), whereas the bonding at CSA sites is about three times weaker thus being similar as on metallic Cu(111). The impact of van der Waals dispersion interactions on molecular adsorption bonding is also addressed. Depending on specifics of the adsorption structure, they strengthen the adsorption bonding by about 0.2–0.5 eV. Due to this specific bonding enhancement, dispersion interactions alter the relative stability of adsorption modes for tetrazole. An atomistic thermodynamics approach was used to construct two-dimensional phase diagrams for all the three molecules. In the viable range of oxygen chemical potential only three phases appear in the phase-diagrams, two of which are the high coverage (1 × 1) molecular phases (one on Cu2O(111) and the other on Cu2O(111)-w/o-CuCUS) and the third is clean Cu2O(111)-w/o-CuCUS. The current results indicate that molecular adsorption at CUS sites is strong enough to compensate the thermodynamic deficiency of stoichiometric Cu2O(111) thus making it more stable than Cu2O(111)-w/o-CuCUS, unless the conditions are too oxygen rich and/or for azole lean. This finding may tentatively suggest that the corrosion inhibition capability of azoles stems from their ability to passivate reactive surface sites.

 Please wait while we load your content...
Please wait while we load your content...