The mechanism of excited-state proton transfer in 1-naphthol–piperidine clusters†
Abstract
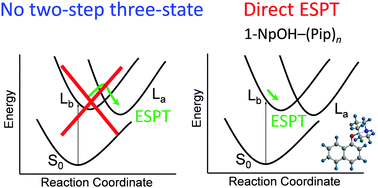
The geometries of 1-naphthol–(piperidine)n (1-NpOH–(Pip)n) (n = 0–3) clusters have been calculated by using density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods to investigate excited-state proton transfer (ESPT) in the low-lying singlet excited states, La and Lb. For the n = 1 cluster, no PT structure was found in Lb and La as well as the ground state, S0. For n = 2, optically accessible Lb from S0 shows the PT structure. We therefore concluded that the threshold size of ESPT is n = 2, which is consistent with previous experimental results. ESPT in 1-NpOH–(Pip)n is simply triggered by optical excitation to Lb. It is essentially different from the 1-NpOH–(NH3)n cluster in which an internal conversion process is required to promote ESPT. From the calculated structures, the importance of the solvation of the π-ring is strongly suggested rather than the proton affinity in ESPT.

 Please wait while we load your content...
Please wait while we load your content...