Observation of the retarded transportation of a photogenerated hole on epitaxial graphene†
Abstract
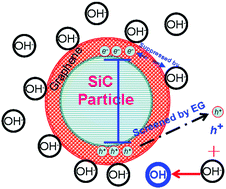
Graphene is usually adopted as an assistant additive for catalysts in photocatalytic processes, because of its ability to accelerate the separation of photogenerated charge carriers. To elucidate the mechanism, hydrogen peroxide is adopted to convert the O2−˙ active species into OH˙ for degradation of an organic dye. If the pH value is less than 7, the concentration of the OH˙ species can be reduced more quickly with the addition of graphene than without, because negatively charged electrons can be transported quickly on graphene. If the pH value is larger than 7, the concentration of OH˙ can be promoted by the catalyst SiC with photogenerated h+ release and reaction with OH−, however the concentration is reduced if the SiC catalyst is covered by a graphene sheet, as it retards h+ release from the SiC substrate. Our findings have provided a certification for the role of graphene in photo-catalytic processes.

 Please wait while we load your content...
Please wait while we load your content...