Heterogeneous photo-Fenton reaction on hematite (α-Fe2O3){104}, {113} and {001} surface facets†
Abstract
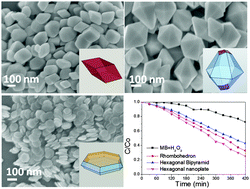
The exposed surface facets play an important role in determining the catalytic performance of nanostructured materials. In this study, we report the synthesis of hematite nanoparticles with three varying morphologies with exposure of well-controlled {104}, {113} and {001} surfaces. The better shape control of hematite particles has provided a direct correlation between the surface facets and the photocatalytic performance. The catalytic photodegradation of MB using hematite nanoparticles reveals that the reaction follows the heterogeneous photo-Fenton process under visible light irradiation. The catalytic performance of hematite surface facets follows the order of {113} > {104} > {001}. Density functional theory (DFT) calculations were conducted to demonstrate the atomic surface structures and the corresponding charge distribution. The results indicate that the catalytic activity depends on surface atom arrangements as well as the number and the type of surface terminated hydroxyl groups bonding to underlying Fe atoms, where low valence states of Fe on {104} and {113} planes have the highest probability to be oxidized by H2O2 and the concurrently generated Fe(3+x)+ sites are more electronegative to accept electrons from activated dye molecules. The findings are of fundamental importance to understand the surface-dependence of photocatalytic properties, thus shedding new light on the catalytic application of hematite particles.

 Please wait while we load your content...
Please wait while we load your content...