Molecular dynamics investigation of separation of hydrogen sulfide from acidic gas mixtures inside metal-doped graphite micropores
Abstract
The separation of poisonous compounds from various process fluids has long been highly intractable, motivating the present study on the dynamic separation of H2S in acidic-gas-mixture-filled micropores. The molecular dynamics approach, coupled with the isothermal–isochoric ensemble, was used to model the molecular interactions and adsorption of H2S/CO2/CO/H2O mixtures inside metal-doped graphite slits. Due to the difference in the adsorption characteristics between the two distinct adsorbent materials, the metal dopant in the graphitic micropores leads to competitive adsorption, i.e. the Au and graphite walls compete to capture free adsorbates. The effects of competitive adsorption, coupled with changes in the gas temperature, concentration, constituent ratio and slit width on the constituent separation of mixtures were systematically studied. The molecule–wall binding energies calculated in this work (those of H2S, H2O and CO on Au walls and those of H2O, CO and CO2 on graphite walls) show good agreement with those obtained using density functional theory (DFT) and experimental results. The z-directional self-diffusivities (Dz) for adsorbates inside the slit ranged from 10−9 to 10−7 m2 s−1 as the temperature was increased from 10 to 500 K. The values are comparable with those for a typical microporous fluid (10−8–10−9 m2 s−1 in a condensed phase and 10−6–10−7 m2 s−1 in the gaseous state). The formation of H-bonding networks and hydrates of H2S is disadvantageous for the separation of mixtures. The results indicate that H2S can be efficiently separated from acidic gas mixtures onto the Au(111) surface by (i) reducing the mole fraction of H2S and H2O in the mixtures, (ii) raising the gas temperature to the high temperature limit (≥400 K), and (iii) lowering the slit width to below the threshold dimension (≤23.26 Å).
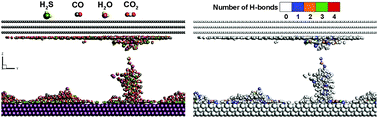

 Please wait while we load your content...
Please wait while we load your content...