Vibrational spectra and structures of SinC clusters (n = 3–8)†
Abstract
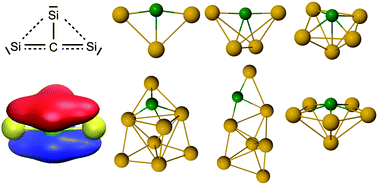
The effects of doping bare silicon clusters with carbon on their physical properties are of fundamental interest for the chemistry of the interstellar medium and the development of novel nanostructures in materials science. Carbon-doped silicon clusters (SinC, n = 3–8) are characterized in the gas phase with infrared-ultraviolet two-color ionization (IR-UV2CI) spectroscopy, mass spectrometry, and quantum chemical calculations. Structural identification is achieved by comparing the measured and calculated vibrational absorption spectra of the low-energy SinC isomers identified by global optimization algorithms. Except for planar Si3C, the most stable SinC clusters have three-dimensional configurations. While the Si3C and Si6C structures are uniquely assigned, several stable isomers of Si4C, Si5C, Si7C, and Si8C may co-exist under the present experimental conditions. Interestingly, some of the structures observed here are different from the ground state structures predicted previously. For the small neutral clusters (n ≤ 5), structures similar to those reported previously for the anions are observed. The highly stable Si3C unit with a nearly linear Si–C–Si motif is identified as characteristic building block in several of the most stable SinC structures. In all identified structures, a large negative charge of almost −2e is located on the C atom, indicating its role as electron donor in the Sin host moiety. The B3LYP/cc-pVTZ level proves reliable in finding the experimentally observed isomers.

 Please wait while we load your content...
Please wait while we load your content...