Comparison studies of rheological and thermal behaviors of ionic liquids and nanoparticle ionic liquids
Abstract
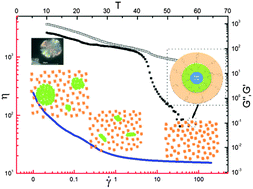
Novel nanoparticle ionic liquids (NILs) are prepared by grafting modified nanoparticles with long-chain ionic liquids (ILs). The NIL behaves like a liquid at ambient temperature. We studied the rheological behavior of the IL and NIL over the range of 10–55 °C and found an extraordinary difference between the IL and NIL: a small content of nanosilica (7%) moderately improves the crystallinity by 7% of the poly(ethylene glycol) (PEG) segment in the IL, and it improves the dynamic moduli significantly (by 5 times at room temperature). It retards the decay temperature (by 10 °C) of the dynamic moduli during heating as well. The thermal rheological hysteresis observed during heating–cooling temperature sweeps is ascribed to the melting–recrystallization of the PEG segments. Meanwhile, the IL and NIL express accelerated crystallization behavior in comparison with the oligomeric anion. For the first time, we find that ILs and NILs are able to form nanoparticle-containing spherulites at room temperature after long time aging.

 Please wait while we load your content...
Please wait while we load your content...