Tandem deprotonation/azide–tetrazole tautomerization of 4,6-diazido-N-nitro-1,3,5-triazin-2-amine in dimethylsulfoxide solutions: a theoretical study†
Abstract
4,6-Diazido-N-nitro-1,3,5-triazin-2-amine (2) is a new promising high-energy organic compound that isomerizes in dimethylsulfoxide (DMSO) solutions to form unknown products with interesting 13C and 15N NMR spectroscopic characteristics. To identify these products, the relative energies and the 13C and 15N chemical shifts were calculated for various prototropic and valence isomers of 2. It was found that this diazide in DMSO solutions exists in equilibrium with the deprotonated form of 5-azido-N-nitrotetrazolo[1,5-a][1,3,5]triazin-7-amine (8). The anion 8 is also observed in DMSO solutions of energetic salts derived from 2 and guanidines or 4H-1,2,4-triazol-4-amines. The tandem transformations of 2 in DMSO solutions found may be of interest from both theoretical and practical points of view, for instance, in the synthesis of new energetic materials, using the reactions of anion 8 with various electrophilic agents.
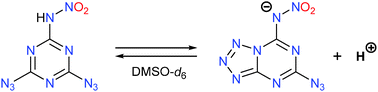

 Please wait while we load your content...
Please wait while we load your content...