On the tunneling instability of a hypercoordinated carbocation†
Abstract
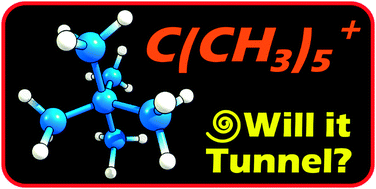
C(CH3)5+, a pentacoordinate carbocation, was recently described as a “fleeting” molecule, that is, stable only at low temperatures. Herein it is shown using theoretical methods that, owing to carbon tunneling, the molecule is not persistent even at 0 K. One possible way to lengthen its lifespan is by deuterium substitution, in spite of the fact that no bond to hydrogen is broken.


 Please wait while we load your content...
Please wait while we load your content...