Gramicidin A disassembles large conductive clusters of its lysine-substituted derivatives in lipid membranes
Abstract
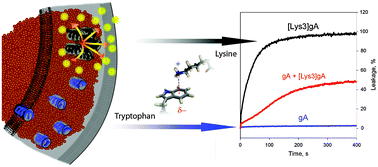
N-terminally substituted lysine derivatives of gramicidin A (gA), [Lys1]gA and [Lys3]gA, but not glutamate- or aspartate-substituted peptides have been previously shown to cause the leakage of carboxyfluorescein from liposomes. Here, the leakage induction was also observed for [Arg1]gA and [Arg3]gA, while [His1]gA and [His3]gA were inactive at neutral pH. The Lys3-containing analogue with all tryptophans replaced by isoleucines did not induce liposome leakage, similar to gA. This suggests that the presence of both tryptophans and N-terminal cationic residues is critical for pore formation. Remarkably, the addition of gA blocked the leakage induced by [Lys3]gA. By examining with fluorescence correlation spectroscopy the peptide-induced leakage of fluorescent markers from liposomes, we estimated the diameter of pores responsible for the leakage to be about 1.6 nm. Transmission electron cryo-microscopy imaging of liposomes with [Lys3]gA showed that the liposomal membranes contained high electron density particles with a size of about 40 Å, suggesting the formation of peptide clusters. No such clusterization was observed in liposomes incorporating gA or a mixture of gA with [Lys3]gA. Three-dimensional reconstruction of the clusters was compatible with their pentameric arrangement. Based on experimental data and computational modeling, we suggest that the large pore formed by [Lys3]gA represents a barrel-stave oligomeric cluster formed by antiparallel double-stranded helical dimers (DH). In a tentative model, the pentamer of dimers may be stabilized by aromatic Trp–Trp and cation–π Trp–Lys interactions between the neighboring DHs. The inhibiting effect of gA on the [Lys3]gA-induced leakage can be attributed to breaking of cation–π interactions, which prevents peptide clusterization and pore formation.

 Please wait while we load your content...
Please wait while we load your content...