Electronic structure and photoelectron spectra of Bn with n = 26–29: an overview of structural characteristics and growth mechanism of boron clusters†
Abstract
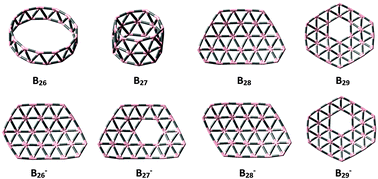
Boron clusters have been of great interest over the last few decades due to their unique chemical and physical properties. In the present work, we performed a theoretical study of geometrical and electronic structures of boron clusters Bn with n = 26–29 in both neutral and anionic states using DFT and MO computational methods. The photoelectron spectra of anionic species were simulated using TDDFT methods. Our results predict that in the neutral state both the B26 and B27 clusters exhibit tubular forms, whereas the larger species B28 and B29 are quasi-planar structures. The anionic species Bn− are more favourable for 2D shapes. More importantly, based on known geometrical characteristics, we now establish a general growth mechanism of boron clusters, which gives us more insight into the formation and existence of boron based nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...